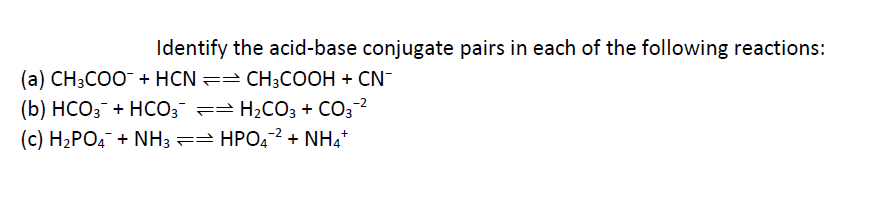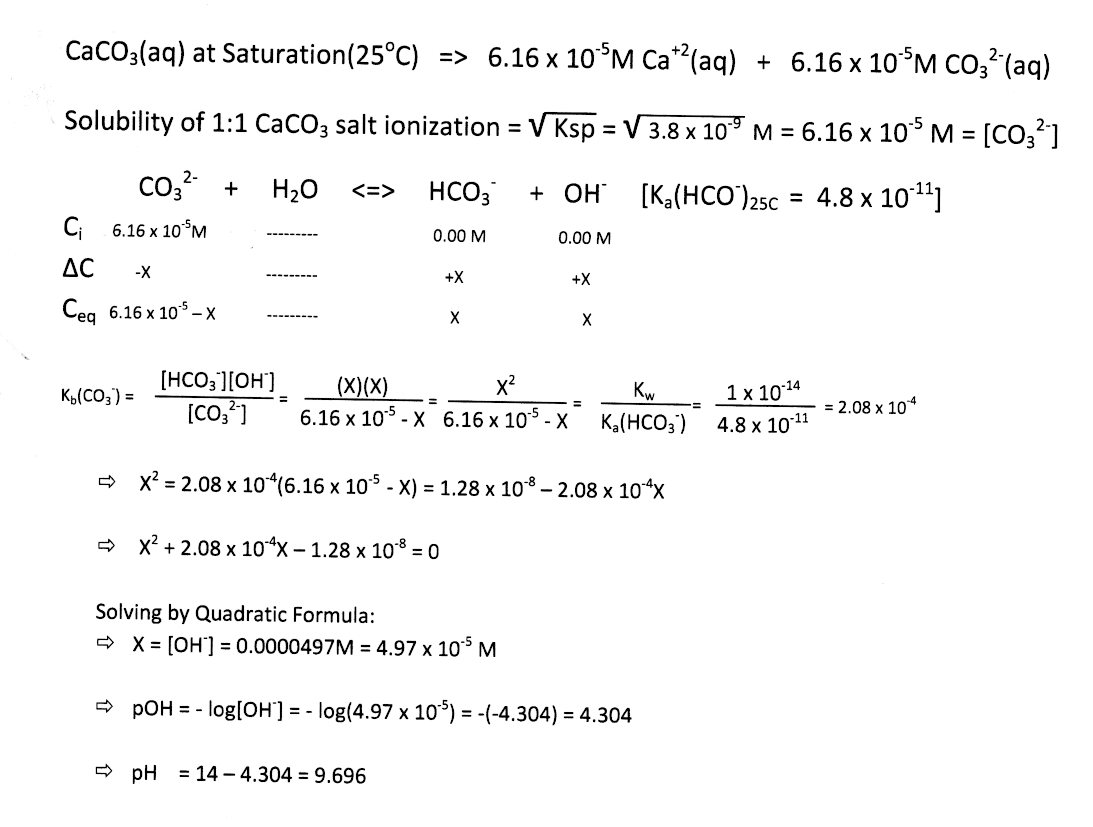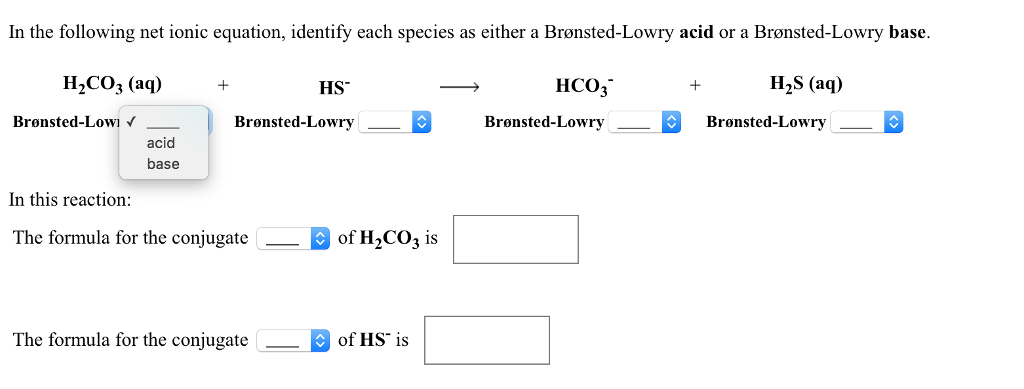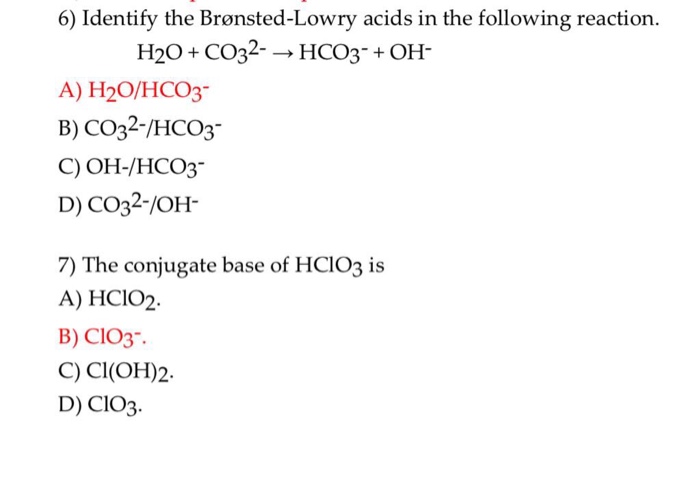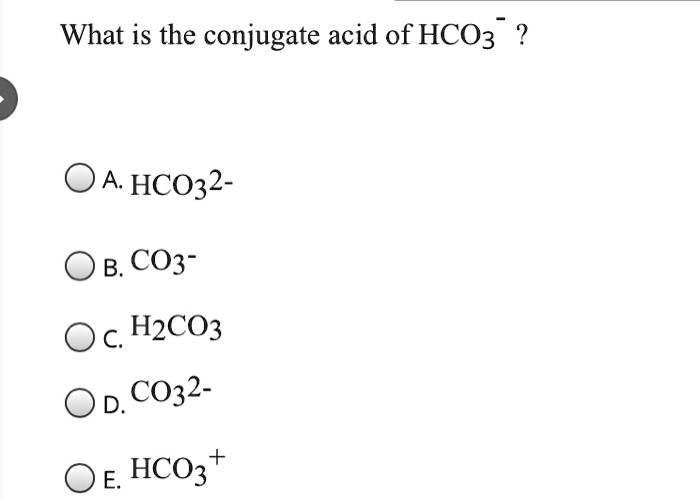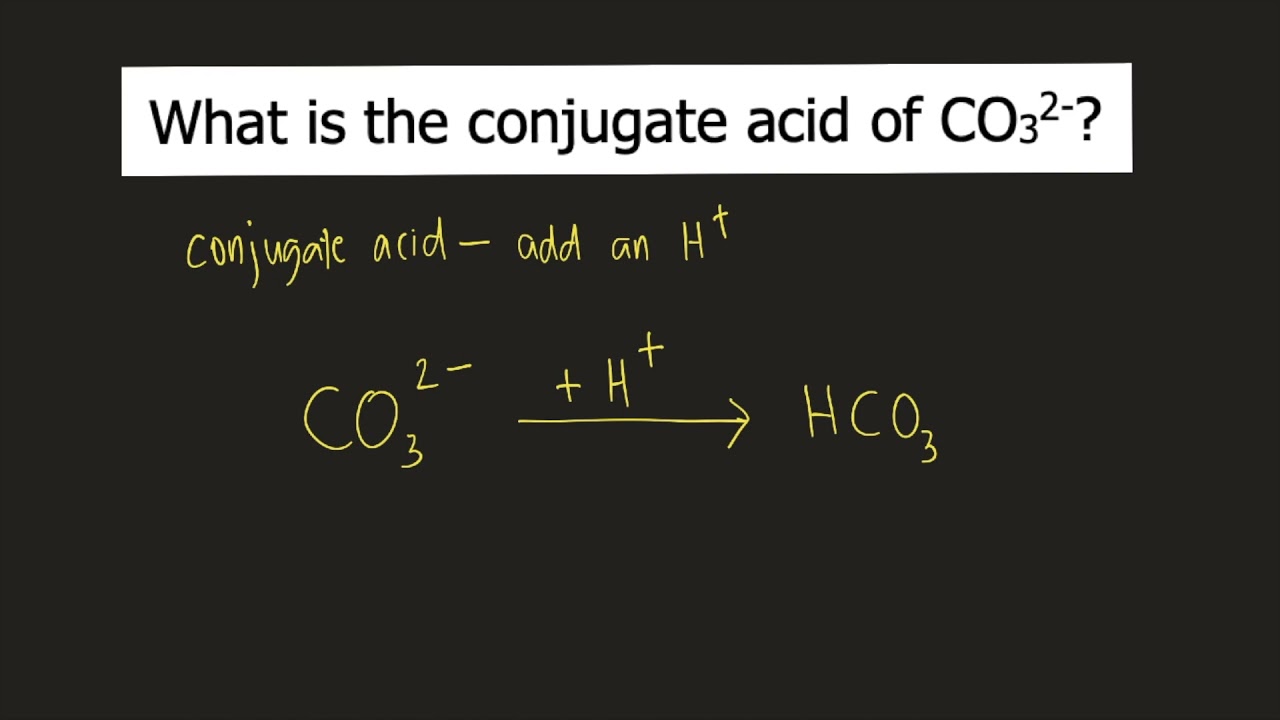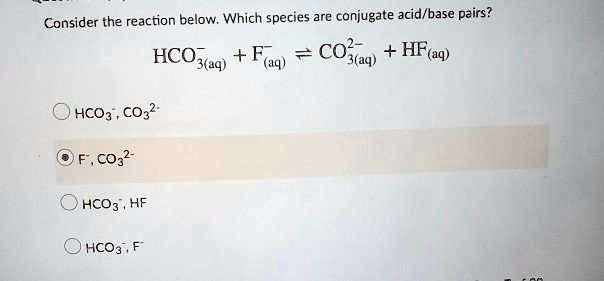
SOLVED: Consider the reaction below: Which species are conjugate acid/base pairs? HCO3(aq) + F(aq) CO3,aq) HF(aq) HCO3 , CO32* F , CO32- HCO3 HCO3 , F

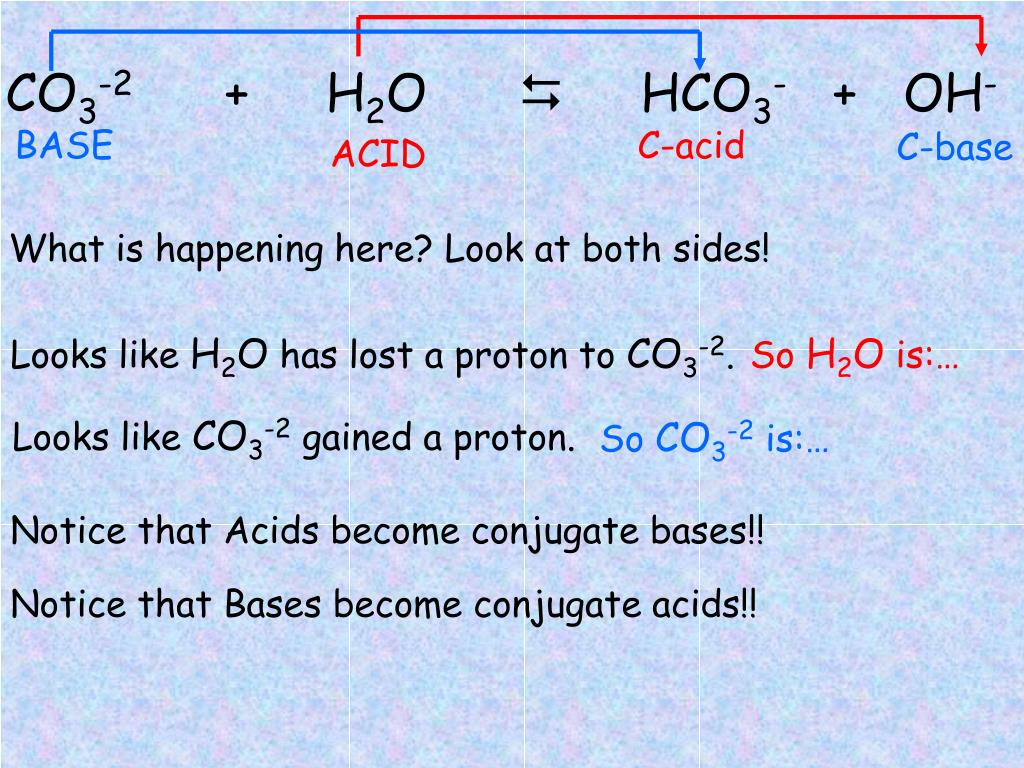
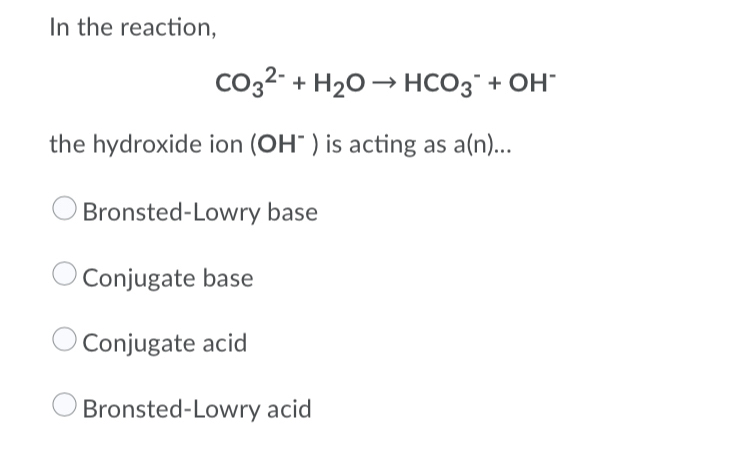
CO3^2- (aq) + H2O (l) \rightarrow (HCO3)- (aq) + OH- (aq) Identify the reactant that is a Bronsted- Lowry Acid and the reactant that is a Bronsted- Lowry Base in the reaction.

The Importance of Acid–Base Equilibria in Bicarbonate Electrolytes for CO2 Electrochemical Reduction and CO Reoxidation Studied on Au(hkl) Electrodes | Langmuir